
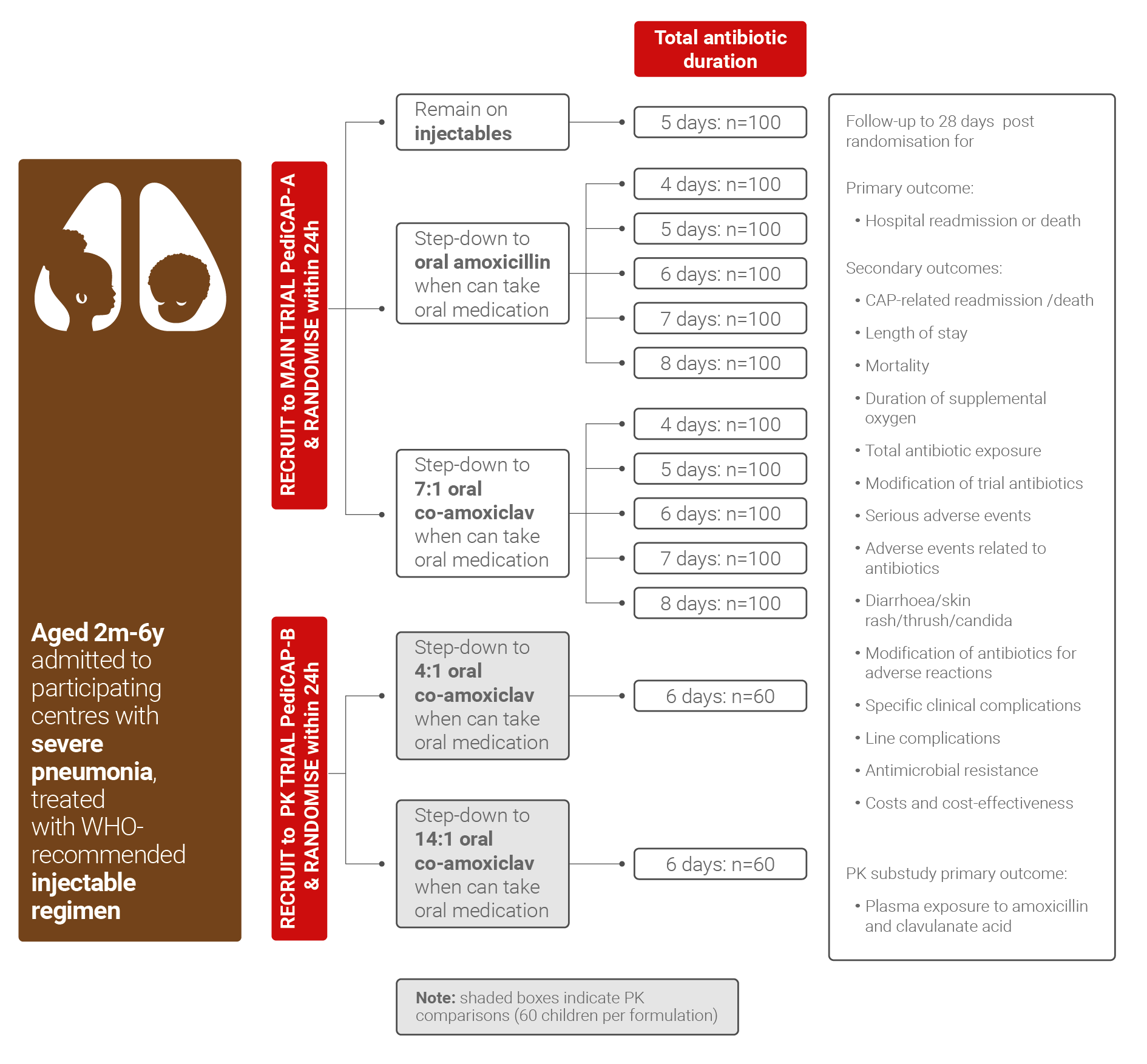
The PediCAP research project will be a randomised trial enrolling 1100 children aged 3 months to 10 years, weighing at least 4 kg, admitted for injectable antibiotic treatment of severe/very severe pneumonia in participating hospitals in South Africa, Uganda, Zambia and Zimbabwe, followed for 4 weeks.
The objectives of the trial are to establish:
- whether superior clinical cure is achieved with co-amoxiclav vs amoxicillin oral step-down therapy
- the optimal antibiotic treatment duration
Microbiology sub-studies will investigate changes in nasopharyngeal and faecal carriage of key colonizing bacteria, including determining the prevalence of antimicrobial resistance, in relation to antibiotic exposure and duration of inpatient stay.
Health economics and equity will assess the costs and cost-effectiveness of different treatment strategies for children with community acquired pneumonia initially requiring inpatient care as well as the impact of different treatment strategies at household level.
Capacity building and networking: the program infrastructure will be harnessed to establish an active community of practice between the research teams and collaborators that will leave the sites with the capability to run, lead and design their own studies beyond this project.
Communications, networking and dissemination: the Consortiumwill develop a strategy for patient and public involvement, communications and capacity strengthening, stakeholder engagement (both internal and external) and the communication and dissemination of outputs and results, including training on communications for researchers.
A pharmacokinetics (PK) sub-study will evaluate and model the pharmacokinetics of solid oral formulation of co-amoxiclav dispersible tablets, in particular considering co-morbidities, such as malnutrition, which may affect drug absorption.