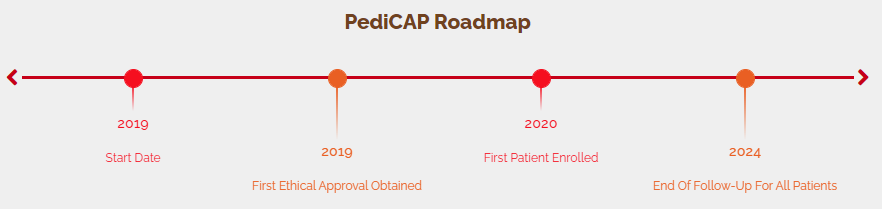
PediCAP is a research project focused on antibiotic therapy of severe and very severe childhood community acquired pneumonia
Through a collaboration between African and European partners it aims to provide data on optimal duration and oral step-down therapy for paediatric community acquired pneumonia.
|
|
|
|
|
|
Project background
Community-acquired pneumonia is common and remains associated with substantial morbidity and mortality, especially in lower and middle-income countries. While standardising care is one important component in reducing mortality from severe pneumonia initially requiring hospitalisation, the antibiotics used are also clearly key. Optimising antibiotic treatment includes defining the right drug, right duration, right delivery and right dose. At present, strong data to support clinical decision-making for each of these factors in very severe/severe childhood pneumonia requiring inpatient care in lower and middle-income countries are lacking.
Goals of PediCAP
- Fill the knowledge gaps on antibiotic therapy of community-acquired pneumonia in pediatric age, in terms of optimal duration, oral step-down schedule evaluating effectiveness, safety and selection of antimicrobial resistance
- Evaluate economic impact of antibiotic therapy in terms of cost-effectiveness
- Implement the infrastructure that links study sites in order to share knowledge, develop study specific and general research skill straining programme and promote capacity development initiatives.
Objectives of PediCAP
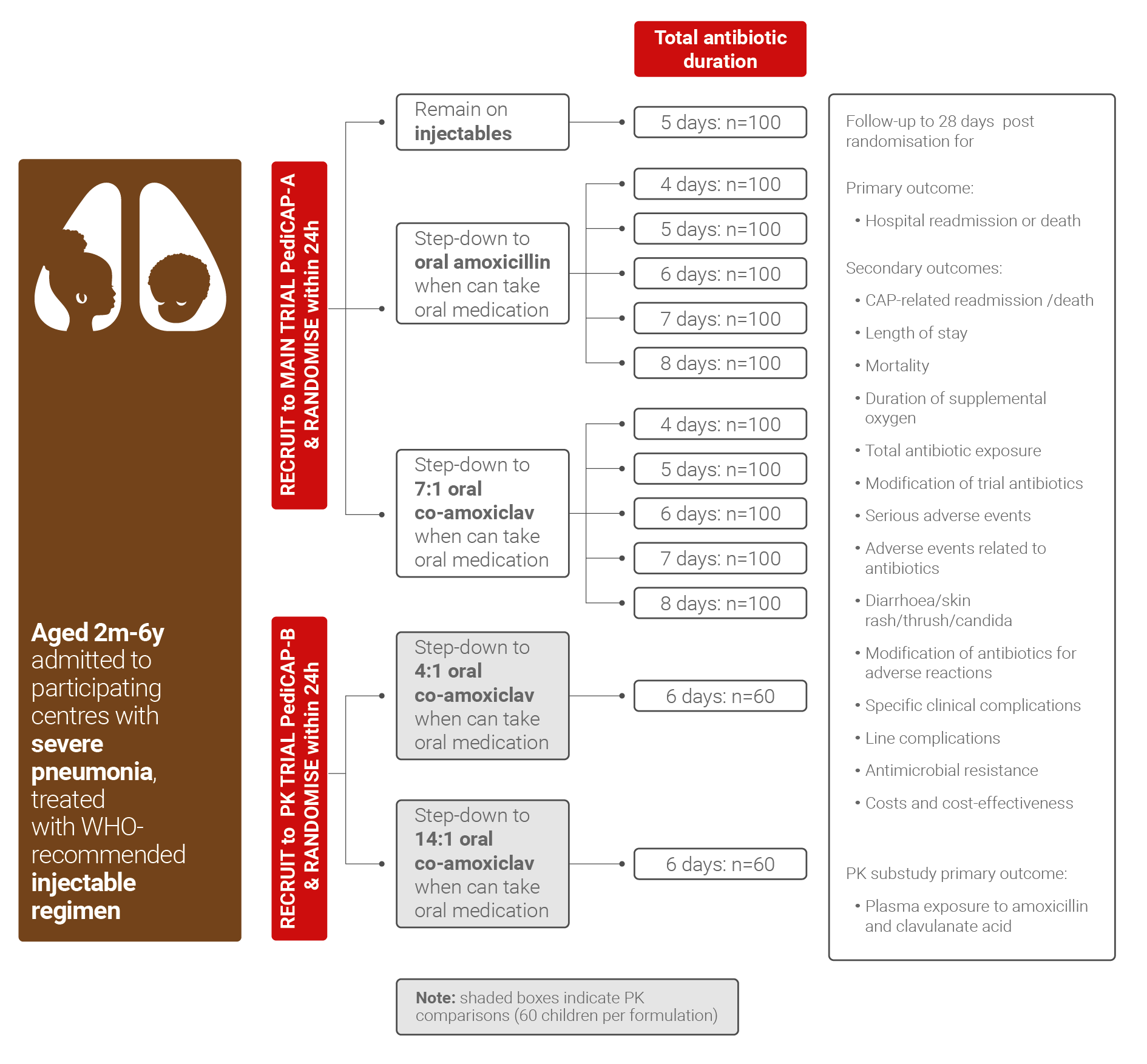
- Optimize antibiotic treatment for childhood pneumonia in low middle-income countries by a randomised trial using an innovative duration - response design
- Understand if the rate of clinical cure is superior with co-amoxiclav vs amoxicillin oral step-down therapy
- Clarify the optimal antibiotic treatment duration that achieves high rates of clinical cure while minimising length of hospital stay, toxicity and antimicrobial resistance
- Detect if optimal antibiotic therapy duration vary by key characteristics, such as age or severity, suggesting that antibiotic duration should be personalised to specific subgroups
Microbiology sub-studies will investigate changes in nasopharyngeal and faecal carriage of key colonizing bacteria, including determining the prevalence of antimicrobial resistance, in relation to antibiotic exposure and duration of inpatient stay.
Health economics and equity will assess the costs and cost-effectiveness of different treatment strategies for children with community acquired pneumonia initially requiring inpatient care as well as the impact of different treatment strategies at household level.
Capacity building and networking: the program infrastructure will be harnessed to establish an active community of practice between the research teams and collaborators that will leave the sites with the capability to run, lead and design their own studies beyond this project.
Communications, networking and dissemination: the Consortiumwill develop a strategy for patient and public involvement, communications and capacity strengthening, stakeholder engagement (both internal and external) and the communication and dissemination of outputs and results, including training on communications for researchers.
A pharmacokinetics (PK) sub-study will evaluate and model the pharmacokinetics of solid oral formulation of co-amoxiclav dispersible tablets, in particular considering co-morbidities, such as malnutrition, which may affect drug absorption.